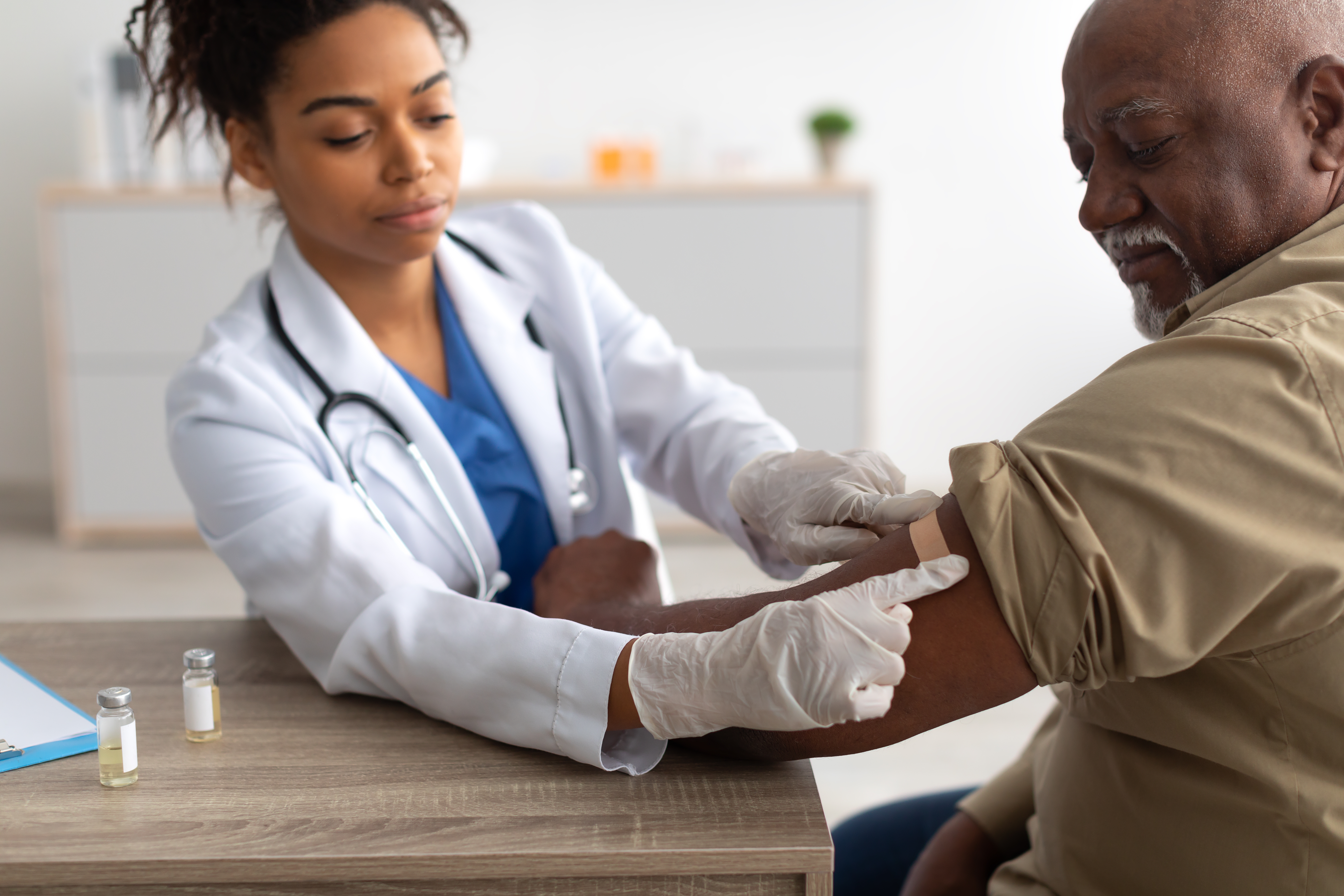
The newest version of the COVID-19 immunization will be ready for the general public in about a week. This is information we need to know.
The Centers for Disease Control and Prevention Director signed off on the recommendation that all Americans aged six months and older receive the updated Covid booster because the number of documented Covid cases and hospitalizations are going up around the county. This comes a day after the U.S. Food and Drug Administration authorized and approved the new shot. The boosters made by Pfizer -Bio-NTech and Moderna were created to target variants that are currently in the air, which are related to XBB, an offshoot of the Omicron variant. The advisory panel made the recommendation–which was then approved by Director Mandy Cohen.
“Vaccinations remain critical to public health and continued protection against serious consequences of COVID-19 including hospitalization and death,” Dr. Peter Marks, Director of the FDA Center for Biologics Evaluation and Research. The new booster shots should be available in about a week or so. They will be delivered to pharmacies and healthcare provider locations around the nation.
President Biden is encouraging all Americans to stay up to date with vaccinations. That means once the new immunization shot is ready about seven days from now, get ready to stick out your arm and get an updated booster.
Meanwhile, in other important COVID-related news you need to know, The Philadelphia Department of Public Health has issued a recall for certain at- home COVID-19 tests on Saturday. Officials stated that city residents who received what they believe to be Flowflex COVID-19 Antigen Home Tests should check the lot number on the box.
If the lot number reads “COV2110012,” officials say to throw the tests away and pick up replacements. People can collect new tests from one of five resource hubs around the city. The Department of Public Health stated that during a routine check–staff
recorded an unknown lot number on Flowflex tests. Upon investigation, officials discovered that this lot number was not listed on the FDA’s shelf-life extension website. Authorities later confirmed that these tests were not authentic, according to the department. Once the discovery was made, tests were removed from distribution, and officials say they are working
to notify those who may have received them. Approximately 105,000 test kits were found to be invalid, and authorities say roughly 4,000 invalid test kits were distributed through the Department of Public Health.
“One of the most important things that Philadelphians can do to prepare their families for the fall and ,…
Thank you for reading Thera Martin article on scoopnewsusa.com. For more on “Living beyond Covid, while Covid is still here“, please subscribe to SCOOP USA Media. Print subscriptions are $75 and online subscriptions (Print, Digital, and VIZION) are $90. (52 weeks / 1 year).